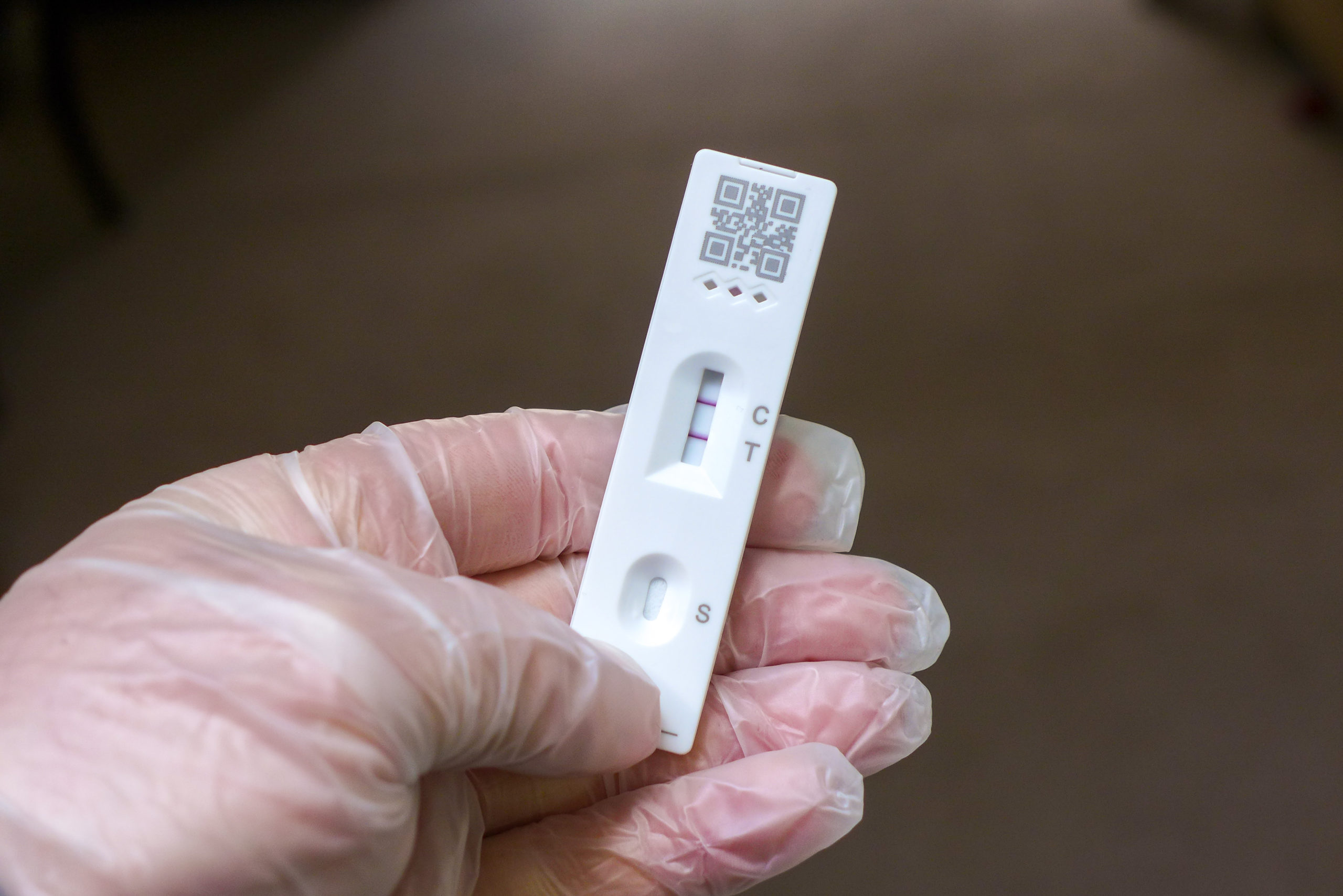
One brand of at-home COVID-19 test is being recalled amid a warning from the Food and Drug Administration that the test could lead to a bacterial infection.
SD Biosensor, Inc. Pilot COVID-19 At-Home Tests, distributed by Roche Diagnostics should be thrown out, according to a new release on the FDA’s website, which lists 44 affected lot numbers.
The FDA reported that about 500,000 tests were distributed to CVS Health. Another 16,000 tests were distributed to Amazon. The FDA said it is not clear how many units were sold.
None of the tests that have been recalled were distributed by the federal government through COVID.gov/tests – Free at-home COVID-19 tests or any other testing program, the FDA said.
Recall Notice – SD Biosensor, Inc. Requests Discontinuation of Use and Disposal of Specific Pilot™ COVID-19 At-Home Tests in the United States Due to Microbial Contamination in the Liquid Buffer Solution https://t.co/xb6GXGrOv8 pic.twitter.com/Ub9evKKHaJ
— U.S. FDA Recalls (@FDArecalls) May 5, 2023
The FDA warning said that anyone who bought a kit that has the affected lot numbers listed on the FDA website should throw the entire kit in the trash. The FDA warns against exposure to a liquid solution in the kit and said that if contact takes place between the liquid and either skin or eyes, the point of contact should be flushed with water.
The liquid in the affected lots has been contaminated with organisms such as Enterococcus, Enterobacter, Klebsiella and Serratia species of bacteria.
Although the liquid is in a tube in the kits, contact is possible while using the kit.
The FDA wanted that infection from the bacteria “may cause illness in people with weakened immune systems or those with direct exposure to the contaminated liquid solution through standard handling, accidental spills, or misuse of the product.”
That’s not all, the FDA said. Test results may also be inaccurate.
The FDA warned that both false negative tests, in which the person has COVID-19 but it is not detected, and false positives, in which an individual does not have COVID-19 but is shown on the test to be infected, are possible.
The FDA also advised doctors who have used the test within the past two weeks to have the individual re-tested.
“SD Biosensor Inc., the manufacturer of the Pilot COVID-19 At-Home Test, informed Roche that this issue was identified during routine quality assurance testing. Potentially harmful bacteria were found in the liquid buffer solution,” Roche said in a statement, according to CBS.
Evie Baik, a representative of SD Biosensor, said in a statement that the infection stems from an issue with raw materials from a supplier.
“To date, no such illness has been reported and to date no impact on performance has been confirmed,” Baik said.
This is not the first recall of the year for at-home COVID-19 tests. In February, more than 56,000 COVID-19 antigen rapid tests were recalled, according to Fox Business.
The Skippack Medical Lab SARS-CoV-2 Antigen Rapid Test Kits produced by Universal Meditech Inc., “potentially could result in inaccurate test results due to lack of performance evaluation by the FDA,” the recall notice said.
This article appeared originally on The Western Journal.













 Continue with Google
Continue with Google